What Is CRISPR?
CRISPR. What is it? And why is the scientific community so fascinated by its potential applications? Starting with its definition, we explain how this technology harnesses an ancient bacteria-based defense system — and how it will impact the world around us today.
Imagine a future where parents can create bespoke babies, selecting the height and eye color of their yet unborn children. In fact, all traits can be customized to one’s preferences: the size of domestic pets, the longevity of plants, etc.
It sounds like the backdrop of a dystopian science fiction novel. Yet some of this is already happening.
Since its initial discovery in 2012, scientists have marveled at the applications of CRISPR (also known as Cas9 or CRISPR-Cas9).
And with a Jennifer Lopez-produced bio-terror TV drama called C.R.I.S.P.R. on the horizon, CRISPR has reached a new peak in interest from outside the scientific community.
CRISPR may revolutionize how we tackle some of the world’s biggest problems, like cancer, food shortages, and organ transplant needs. Recent reports even examine its use as a highly efficient disease diagnostics tool. But, as with any new technology, it may also cause new unintended problems.
Changing DNA — the code of life — will inevitably come with a host of important consequences. But society and industry can’t have this conversation without understanding the basics of CRISPR.
In this explainer, we dive into CRISPR, from a simple explanation of what exactly it is to its applications and limitations.
TABLE OF CONTENTS
What is CRISPR?
CRISPR is a defining feature of the bacterial genetic code and its immune system, functioning as a defense system that bacteria use to protect themselves against attacks from viruses. It’s also used by organisms in the Archaea kingdom (single-celled microorganisms).
The acronym “ CRISPR” stands for Clustered Regularly Interspaced Short Palindromic Repeats. Essentially, it is a series of short repeating DNA sequences with “spacers” sitting in between them.
In short, bacteria use these genetic sequences to “remember” each specific virus that attacks them.
They do this by incorporating the virus’ DNA into their own bacterial genome. This viral DNA ends up as the spacers in the CRISPR sequence. This method then gives the bacteria protection or immunity when a specific virus tries to attack again.
Accompanying CRISPR are genes that are always located nearby, called Cas (CRISPR-associated) genes.
Once activated, these genes make special proteins known as enzymes that seem to have co-evolved with CRISPR. The significance of these Cas enzymes is their ability to act as “molecular scissors” that can cut into DNA.
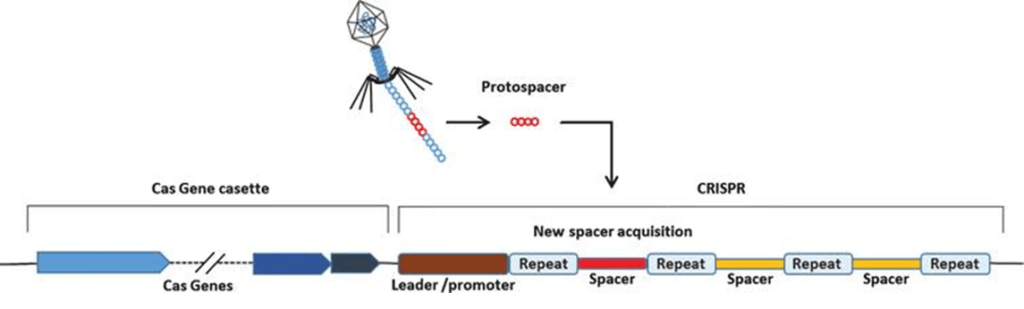 Image: Generalized CRISPR locus. Credit: Lee S, et al. Journal of Cellular Biotechnology
Image: Generalized CRISPR locus. Credit: Lee S, et al. Journal of Cellular Biotechnology
To recap: in nature, when a virus invades bacteria, its unique DNA is integrated into a CRISPR sequence in the bacterial genome. This means that the next time the virus attacks, the bacteria will remember it and send RNA and Cas to locate and destroy the virus.
While there are other Cas enzymes derived from bacteria that cut out viruses when they attack bacteria, Cas9 is the best enzyme at doing this in animals. The widely-known term CRISPR-Cas9 refers to a Cas variety being used to cut animal (and human) DNA.
In harnessing this technology, researchers have added a new step: after DNA is cut by CRISPR-Cas9, a new DNA sequence carrying a “fixed” version of a gene can nestle into the new space. Alternatively, the cut can altogether “knock out” of a particular unwanted gene — for example, a gene that causes diseases.
One way to think about CRISPR-Cas9 is to compare it to the Find & Replacefunction in Word: it finds the genetic data (or “word”) you want to correct and replaces it with new material. Or, as CRISPR pioneer Jennifer Doudna puts it in her book A Crack In Creation: Gene Editing and the Unthinkable Power to Control Evolution, CRISPR is like a Swiss army knife, with different functions depending on how we want to use it.
CRISPR research has moved so fast that it’s already gone beyond basic DNA editing. In December 2017, the Salk Institute designed a “handicapped” version of the CRISPR-Cas9 system, capable of turning a targeted gene on or off without editing the genome at all. Going forward, this kind of process could ease the concerns surrounding the permanent nature of gene editing.
How it works
These are the 3 key players that help the CRISPR-Cas9 tech do its work:
- Guide RNA: a piece of RNA (a genetic cousin of DNA) that locates the targeted gene. This is engineered in a lab.
- CRISPR-associated protein 9 (Cas9): the “scissors” that snip the undesired DNA out
- DNA: the desired piece of DNA that is inserted after the break
Below, we illustrate how these parts come together to create a potential therapy.
Please click to enlarge.
The guide RNA serves as the “GPS coordinates” for finding the piece of DNA you want to edit and zeroes in on the targeted part of the gene. Once located, Cas9, the scissors, makes a double stranded break in the DNA, and the DNA you want to insert takes its place.
The implications for this are vast.
Yes, this technology will disrupt medical treatment. But beyond that, it could also transform everything from the food we eat to the chemicals we use as fuel, since these may be engineered through gene technology as well.
Feng Zhang, PhD, from the Broad Institute of MIT and Harvard, described CRISPR using a helpful nursery rhyme . We can imagine a certain DNA sequence that is fixed in this way:
Twinkle Twinkle BigStar → Twinkle Twinkle LittleStar
In this process:
- Guide RNA locates the error or the mutation: the word “Big.”
- Cas9 enzyme makes the break before and after the word “Big.”
- A bioengineered vehicle inserts the correct piece of DNA, in this case, the word “Little.”
Key researchers in crispr’s discovery
The CRISPR sequence was first discovered in 1987. But its function would not be discovered until 2012.
Key people involved in the initial discovery of the bacterial CRISPR-Cas9 system’s function include Jennifer Doudna, PhD at University of California, Berkeley, and French scientist Emmanuelle Charpentier, PhD. Through their strategic collaboration, they ushered in a new era of biotechnology.
Another important figure is Feng Zhang, PhD, who was instrumental in figuring out CRISPR’s therapeutic applications using mice and human cells in 2013. Harvard geneticist George Church, PhD also contributed to early CRISPR research with Zhang.
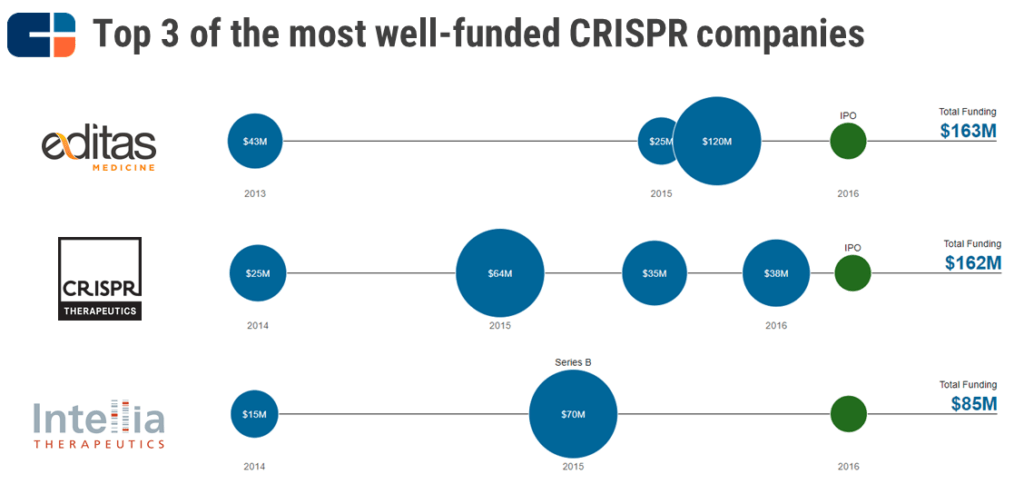
All four researchers went on to play crucial roles in setting up some of the most well-funded CRISPR therapeutic startups, including Editas Medicine, CRISPR Therapeutics, and Intellia Therapeutics. All 3 of these companies IPO’ed in 2016 and are in the drug discovery/pre-clinical stage of testing their respective CRISPR therapeutic candidates for various human diseases.
Gene editing before CRISPR
Before CRISPR was heralded as the gene editing method, two other gene-editing techniques dominated the field: Zinc Finger Nucleases (ZFNs) and Transcription Activator-Like Effector Nucleases (TALENs). Research efforts using these tools are still ongoing.
Like CRISPR, these tools can each cut DNA. Thought they are generally more difficult to make and use, these tools do offer their own advantages:
- ZFNs have an easier delivery process to the targeted gene of choice.
- TALENs appear to have a higher precision rate than CRISPR and may cause less “off-target mutations” (i.e. unintended consequences) as a result of gene editing.
Each also has vital therapeutic applications.
Biotech company Cellectis uses the TALEN gene editing technology to make CAR-T therapies for leukemia, while Sangamo BioSciences makes ZFNs that can disable a gene known to be key in HIV infection. Notably, each of these companies hold key IP rights to these specific gene-editing methods, which could make it difficult for other biotech companies to use these tools.
Meanwhile, CRISPR has certainly stolen the spotlight as of late, due to its efficiency, flexibility, and cheap price tag. It’s plausible that CRISPR could face similar IP issues — and there are already some IP controversies going on — but with such vast applications for this system, research on multiple fronts seems to be moving forward fast.
Applications of CRISPR
Every industry can harness CRISPR as a tool: it can create new drug therapies for human diseases, help farmers grow pathogen-resistant crops, create new species of plants and animals — and maybe even bring back old ones.
Animal-related research
Since the initial discovery of CRISPR as a gene-editing mechanism, the list of applications has grown rapidly. Though still in early stages, “animal models” (i.e. lab animals) have provided key insights into how we may be able to manipulate CRISPR.
Mice have been especially telling when it comes to CRISPR’s therapeutic potential. As mammals sharing more than 90% of our human genes, mice have been used as ideal test subjects.
Experiments on mice have shown that CRISPR can disable a defective gene associated with Duchenne muscular dystrophy (DMD), inhibit the formation of deadly proteins involved in Huntington’s disease, and eliminate HIV infection.
In 2015, Chinese scientists created two super muscular beagles by disabling the myostatin gene, which directs normal muscle development. In the absence of the gene, the beagles displayed “muscular hypertrophy,” creating dogs which were visibly much more muscular than non-genetically modified ones.
Other CRISPR animal trials have ranged from genetically engineering long-haired goats for higher production of cashmere to breeding hornless cows to avoid the painful process of shearing horns off.
Human-related research
Compared to research involving animals, CRISPR trials that edit human DNA have moved more slowly, largely due to the ethical and regulatory issues at play.
Given the permanent nature of altering a human’s genome, the FDA is approaching CRISPR cautiously. Some scientists have even proposed a moratorium on CRISPR trials until we have more information on the potential impact on humans.
United States & EuropeIn the US and Europe, 2018 will be the year for CRISPR human trials.
Currently (as of 2/13/18), the University of Pennsylvania is awaiting the FDA’s final approval to start a study that would evaluate the safety of using CRISPR for patients with multiple myeloma, melanoma, and sarcoma.
Europe may also see its first human CRISPR study in 2018 with CRISPR Therapeutics‘ study focused on a blood disorder known as beta-thalassemia, which results in abnormal red blood cell production.
While clinical trials involving patient participation are still awaiting regulatory approval, CRISPR has already been applied to both viable and non-viable human embryos.
For example, in August 2017, a team lead by reproductive biologist Shoukhrat Mitalipov of Oregon Health and Science University received private funding to use CRISPR-Cas9 to target a mutation in viable human embryos that causes the thickening of heart muscles. The altered embryos came back 72% mutation-free in the lab (higher than the usual 50% chance of inheritance).
Some critics say the gene editing of embryos is unethical, even if the edited embryos are not destined for transfer and implantation. This type of testing currently does not receive federal funding, but instead relies on private donor funding.
ChinaOn the other side of the world, Chinese researchers operate under a different regulatory framework. Some hospital ethics committees can approve studies in as little as one day, with no need to seek approval from a federal agency.
Since 2015, China has been conducting human trials using CRISPR to combat various cancers, HIV, and HPV. It is the only country in the world to conduct human trials thus far.
According to ClinicalTrials.Gov, there are 10 active or upcoming CRISPR therapy trials in China, targeting advanced cancers like stage 4 gastric and nasopharyngeal carcinomas. So far results are only anecdotal, and while some participants’ tumors shrank, no formal results have been made available.
Although possible long-term side effects aren’t fully understood, CRISPR is already an option for some patients in China who have exhausted all of the conventional treatments.
affected industries
Potential high impact industries for CRISPR include medicine, food, agriculture, and the industrial biotech space. Because the CRISPR-Cas9 gene-editing system is so easy to make and use, researchers from a range of scientific disciplines can access it to genetically engineer the organism of their choice.
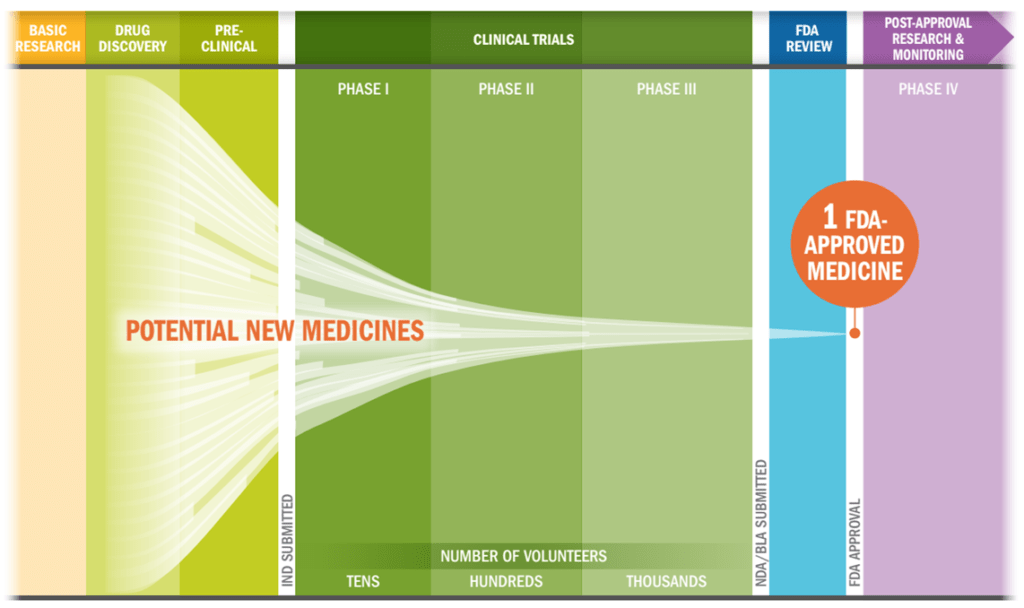
Pharmaceuticals & biotechnologyThe future of medicine will be written with CRISPR.
The current drug discovery process is long, given the need to ensure patient safety and gain a thorough understanding of unintended effects. Moreover, current US regulatory policies often result in a decades-long development process.
However, teams using CRISPR can bring customized therapies to market more quickly than was previously dreamed, speeding up the traditional drug discovery process.
Timeline of drug development. Credit: PhRMA
CRISPR’s cheap price tag and flexibility allows accurate and fast identification of potential gene targets for efficient pre-clinical testing. Because it can be used to “knock out” different genes, CRISPR gives researchers a faster and more affordable way to study hundreds of thousands of genes to see which ones are affected by a particular disease.
Of course, along with providing a more streamlined drug development process, CRISPR offers the possibility of new ways to treat patients.
For example, monogenic diseases — diseases caused by a mutation in a single gene — present an attractive starting point for CRISPR trials. The nature of these illnesses provides an exact target for the treatment: the problematic mutation on a single gene.
Blood-based, single-gene diseases like beta-thalassemia or sickle cell are also great candidates for CRISPR therapy, because of their ability to be treated outside of the body (known as ex-vivo therapy). A patient’s blood cells can be taken out, treated with the CRISPR system, then put back into the body.
Food & agricultureAn early application of CRISPR was pioneered by yogurt company Danisco in the 2000s, when scientists used an early version of CRISPR to combat a k ey bacterium found in milk and yogurt cultures (streptococcus thermophilus) that kept getting infected by viruses. At that point, the ins and outs of CRISPR were still unclear.
Fast forward to today, when climate change will further increase the need to use CRISPR to protect the food and agriculture industries against new bacteria. For example, cacao is becoming difficult to farm as growing regions get hotter and drier. This environmental change will further exacerbate the damage done by pathogens.
“If you’ve eaten yogurt or cheese, chances are you’ve eaten CRISPR-ized cells.”
— Rodolphe Barrangou, former Danisco scientist & Editor-in-Chief of The CRISPR Journal
To combat this issue, the Innovative Genomics Institute (IGI) at UC Berkeley is applying CRISPR to create disease-resistant cacao. Leading chocolate supplier MARS Inc. is supporting this effort.
Gene editing can make farming more efficient. It can curb global food shortages for staple crops like potatoes and tomatoes. And it can create resilient crops, impervious to droughts and other environmental impacts.
Regulators have shown little resistance to gene-edited crops, and the United States Department of Agriculture (USDA) in particular is not regulating the space. This is largely because when CRISPR is applied to crops, t here’s no foreign DNA being added: CRISPR is simply used to edit a crop’s own genetics to select for desirable traits.
In 2016, the white button mushroom, modified to be resistant to browning, became the first CRISPR-edited organism to bypass USDA. In October 2017, it was announced that agriculture company DuPont Pioneer and the Broad Institute would collaborate for agriculture research using their CRISPR-Cas9 intellectual property.
In January, biotech company Yield10 Biosciencegot approval for its CRISPR-edited plant Camelina sativa (false flax), which has enhanced omega-3 oil and is used to make vegetable oil and animal feed.
These are indications that new breeds of crops could reach markets much faster than previously thought. Without USDA oversight, these items and other food products could go into production relatively quickly.
This will impact the food we eat, as food items are edited to carry more nutrients or to last longer on grocery shelves.
Another area currently generating buzz is the production of leaner livestock.
In October 2017, scientists at the Chinese Academy of Sciences in Beijing used CRISPR to genetically engineer pig meat that had 24% less body fat.
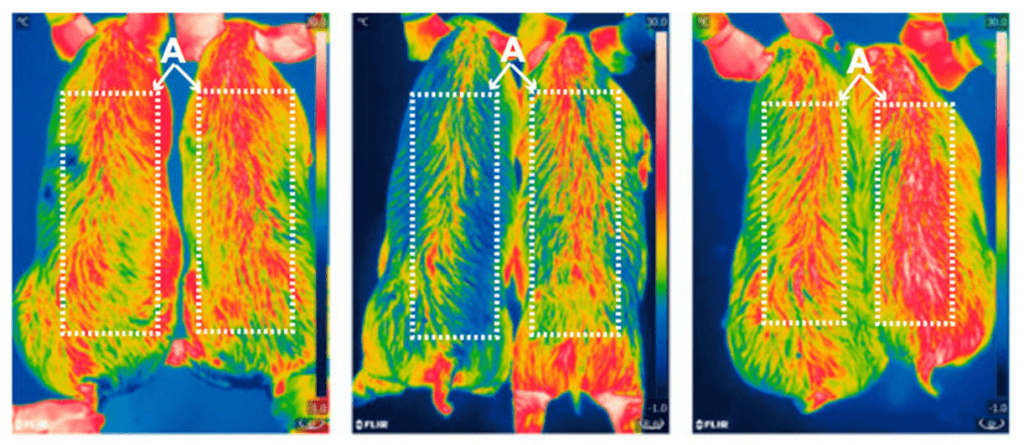 Image: Infrared pictures of 6-month-old pigs at zero, two, four hours after cold exposure. Gene edited pigs are on the right side of the images. Credit: Zheng et al./PNAS
Image: Infrared pictures of 6-month-old pigs at zero, two, four hours after cold exposure. Gene edited pigs are on the right side of the images. Credit: Zheng et al./PNAS
Researchers did this by inserting a mouse gene into pig cells in order to better regulate body temperature. Although this example technically makes the result a GMO product, it may not be too long before pigs’ genes are used for the same purpose.
Future versions of this technology applied to human nutrition will be one area to look out for.
Industrial biotechnologyAnother key, but less obvious, use of CRISPR lies is in the industrial biotech space. By re-engineering microbes using CRISPR, researcher can create new materials.
How is this relevant to society at large?
From an industrial standpoint, this is big for modifying and creating new chemical products. We can alter microbes to increase diversity, create new bio-based materials, and make more efficient biofuels. From active chemicals in fragrances to those involved in industrial cleaning, CRISPR could have a great impact here by creating new and more efficient biological materials.
Jennifer Doudna’s first CRISPR startup, Caribou Biosciences, was founded in 2011 for non-therapeutic research purposes across industries. It is one of the key companies providing various industries with the tools to use CRISPR for a range of purposes.
LIMITATIONS
CRISPR’s list of potential benefits is a long one. But the technology also brings with it a number of limitations.
Possible unintended effects and all the unknown variables are some of the drawbacks to this new technology, while new ethical questions and controversies are also emerging as human trials near.
side effect limitations
When using CRISPR for human therapies, safety is the biggest issue. As with any new form of technology, researchers are unsure of the entire range of CRISPR’s effects.
Off-target activity is the main concern here. A single gene edit could cause unintended activity somewhere else in the genome. A possible consequence of this is abnormal growth of tissues, leading to cancer. As more research uncovers new details, this could result in more refined, precise gene targeting.
Another issue is the possibility of mosaic generation. After a CRISPR treatment, a patient could have a mix of both edited and unedited cells — a “mosaic.” As cells continue to divide and replicate, some cells may get repaired, while others won’t.
Finally, immune system complications mean that these interventions and therapies may trigger an undesired response from a patient’s immune system. Early research shows the immune system may dispose of Cas enzymes before they achieve their purpose, or may have an averse reaction resulting in side effects like inflammation. (In 1999, a patient in the US died of a severe immune reaction, instilling more caution in researchers when it comes to CRISPR trials.)
However, all three of these limitations have some possible solutions.
Different enzymes (“molecular scissors”) or more precise delivery vehicles can reduce off-target activity. If modified stem cells in egg or sperm (i.e. cells that can become every cell in the human body) are edited, mosaics can be avoided.
With the immune system issue, researchers can isolate different Cas proteins from more obscure bacterial strains that humans don’t already have an adaptive immunity to in order to circumvent an unwanted immune response. Meanwhile, ex- vivo therapies, where scientists take a patient’s blood cells out of the body and treat them before infusing them back in, can also help bypass the immune system.
Biological alternatives
One potential big limitation for CRISPR is that CRISPR-Cas9 system lacks surgical precision. The Cas enzyme cuts both strands of the DNA double helix, and this “double-stranded break” creates worries over the precision of the cut.
“Repairing a defective gene would be like finding a needle in a haystack and then removing that needle without disturbing a single strand of hay in the process.” -Jennifer Doudna
While currently the Cas9 enzyme gets the most attention as the enzyme doing the “cutting,” scientists are actively pursuing alternatives to find better candidates.
Alternative options include a smaller version of Cas9, or a different enzyme entirely: Cpf1, which has become popular due to its easy transport to the targeted DNA location.
Besides using other Cas enzymes, alternate delivery vehicles for therapeutic genes are another option. Harmless engineered viruses can carry therapeutic genes to the site of mutation, while lipid nanoparticles can avoid immune system detection, avoiding an immune reaction. Both options present promising avenues of research.
Controversies
When technology can alter the code of life, its implications are far-reaching — as are its controversies. Here we outline a few of the main controversies surrounding CRISPR.
Design-your-own-baby?
If we know where a certain gene is located, CRISPR allows us to manipulate it in many ways.
By this logic, pet owners could design the dog they want with a specified coat color and size. What’s more, parents could hypothetically tinker with a gene that controls height or eye color to “design” their children. If we could isolate the genes associated with intelligence, that too could be manipulated.
While critics say this technology should only be reserved for therapeutic needs, the fast-paced development of CRISPR doesn’t seem to be slowing down any time soon, and gene-edited organisms have already been created for non-therapeutic purposes.
In 2015, the Beijing Genome Institute (BGI) created “micro-pigs” by removing a gene associated with growth. At just 30 pounds, this was quite a departure from pigs’ normal weight of 100 pounds. BGI originally wanted to sell each micro-pig for an estimated $1,600, with customized size and color options for consumers. Plans were eventually scrapped in 2017.
Although BGI used TALENs and not CRISPR to edit the pigs’ genes, CRISPR poses a similar concern when it comes to “designing” future pets. T his designer application could represent a permanent, irreversible turning point in the direction of animal and human evolution.
bringing back extinct animals
Woolly mammoths were last seen 3,600 years ago. If we could bring back these ancient creatures, should we? What would be the purpose? Whether for mere curiosity or for valid scientific purposes, this area is ripe for controversy.
Simply put, de-extinction is the effort to bring back extinct animals. The process involves taking the embryo of the closest living relative of the extinct animal and using CRISPR-Cas9 to insert the extinct species’ DNA, so an extinct animal could once again roam the planet.
Such initiatives are already being pursued by different scientific groups and organizations. Notably, a project by The Long Now Foundation, called “Revive & Restore,” aims to bring back extinct animals like the passenger pigeon and the woolly mammoth. Geneticist George Church of Broad Institute has been instrumental in bringing this project to fruition.
 Credit: Revive & Restore
Credit: Revive & Restore
The woolly mammoth is of special interest to many scientists. Editing the Asian elephant genome to insert genes from conserved wooly mammoth tissue remains could undo extinction, or perhaps more likely result in a hybrid version of the ancient creature.
In this case there are also climate considerations at play: some researchers believe the mammoth’s effect on far-northern forests could bring back ideal conditions to contribute to permafrost and reduce carbon release into the atmosphere.
Advocates of de-extinction also say that humans were directly responsible for many species becoming extinct, and therefore, we should make a concerted effort to reverse the trend.
Critics raise concerns that manipulating nature like this will possibly bring more harm than good, potentially creating species that wreak havoc on an environment that has already been irrevocably changed by the rise of homo sapiens.
Irreversible genetic changes for generations
Using CRISPR for “germline modifications” is making the scientific community nervous.
Somatic modifications are done on body cells such as skin, brain, muscle, and heart cells, and the modifications do not get passed on to future generations. Germline modifications, on the other hand, are done in genes carried in reproductive egg or sperm cells — and thus will be inherited by future generations.
Working with germline cells raises the question: can we ethically choose the genetic changes we want for future unborn generations?
The consequences of making genetic changes to germline cells were one reason why, until recently, researchers were only using non-viable human embryos for CRISPR studies. Then, in March 2017, the first CRISPR experiment on viable human embryos was conducted on six embryos in China. Notably, t he experiment showed a higher success rate of gene editing than previous experiments with non-viable embryos.
Despite the controversy surrounding the issue, the clear benefit of altering germline cells is that a disease can be contained or prevented from expressing itself as an individual develops. Germline therapies are also guaranteed to reach every cell in a patient’s body.
Through a germline CRISPR gene edit, an adult could hypothetically never suffer from cancer, even if he or she has a genetic predisposition to it.
Biohackers
Biohackers say they want to leverage CRISPR to move the traditionally lengthy R&D process along on a faster timeline, raising the question of whether — and how — they should be regulated.
 Image: DIY Bacterial Gene Engineering CRISPR Kit, Credit: The Odin
Image: DIY Bacterial Gene Engineering CRISPR Kit, Credit: The Odin
Recent crowdfunding campaigns by Do-It-Yourself (DIY) biohackers have received a lot of attention. The Odin, a biohacking startup, sells DIY Bacterial CRISPR Kits on its website for a retail price of $159. Odin’s CEO, Josiah Zayner, self-injected himself with a CRISPR-modified muscle growth gene at a synthetic biology conference in San Francisco in October 2017.
For the moment, this type of biohacking remains unregulated, as it was self-injection and not experimentation on other people. However, the FDA does prohibit sales that tout this kind of self-injection for therapeutic purposes.
The future of CRISPR
Future applications of CRISPR technology are essentially as infinite as the forms of life itself. While current initiatives are mostly geared toward therapeutics or food tech, there are also some less prominent but very real applications of the CRISPR-Cas9 system.
Xenotransplantation
Xenotransplantation is the act of transplanting another animal’s cells, tissues, or organs to a human recipient.
W ith the high demand for organ transplants and no supply to match it, xenotransplantation (FDA definition here ) could be the solution for many sick patients waiting for an organ transplant.
The process could look something like this:
- Scientists inject human stem cells inside a living pig.
- These human stem cells grow and differentiate into a specific type of cell inside that pig.
- The stem cells are “educated” through Cas9 and directed to become a certain cell type (i.e. heart, liver, pancreas, etc.).
The California-based Salk Institute created a lot of noise in the scientific community when it announced that it had made a chimera organism, made of both pig and human cells, in January 2017.
 Xenotransplantation, Credit: eGenesis
Xenotransplantation, Credit: eGenesis
Xenotransplantation has been previously tested in mice with rat stem cells: CRISPR-Cas9 was used to turn off the gene that makes pancreas in mice and instead, rat stem cells were inserted into mouse embryos. The mice, as programmed, went on to grow rat pancreas.
Later on, researchers inserted human stem cells (known as inducible pluripotent stem cells, or iPSCs) in pig embryos. The study was stopped at 4 weeks due to safety and effectiveness concerns. Researchers did note that some of the stem cells specialized in these pig embryos, which meant they were turning into the beginnings of human tissues. While this happened at a lower success level than was seen with rat pancreases in mice embryos, it was still quite a scientific feat.
Co-founded by George Church, eGenesis is also working in this area, with the goal of creating pig organs for human use. In August 2017, scientists modified over 60 genes in pig embryos to get rid of retroviruses that human bodies would reject in a transplant scenario.
If researchers figure out a way to grow human cells in a living animal, they could create organs specially matched to a patient. Each patient’s stem cells could give rise to his or her own engineered organ constituted of his or her own unique DNA, reducing the risk of rejection and other consequences of organ failure.
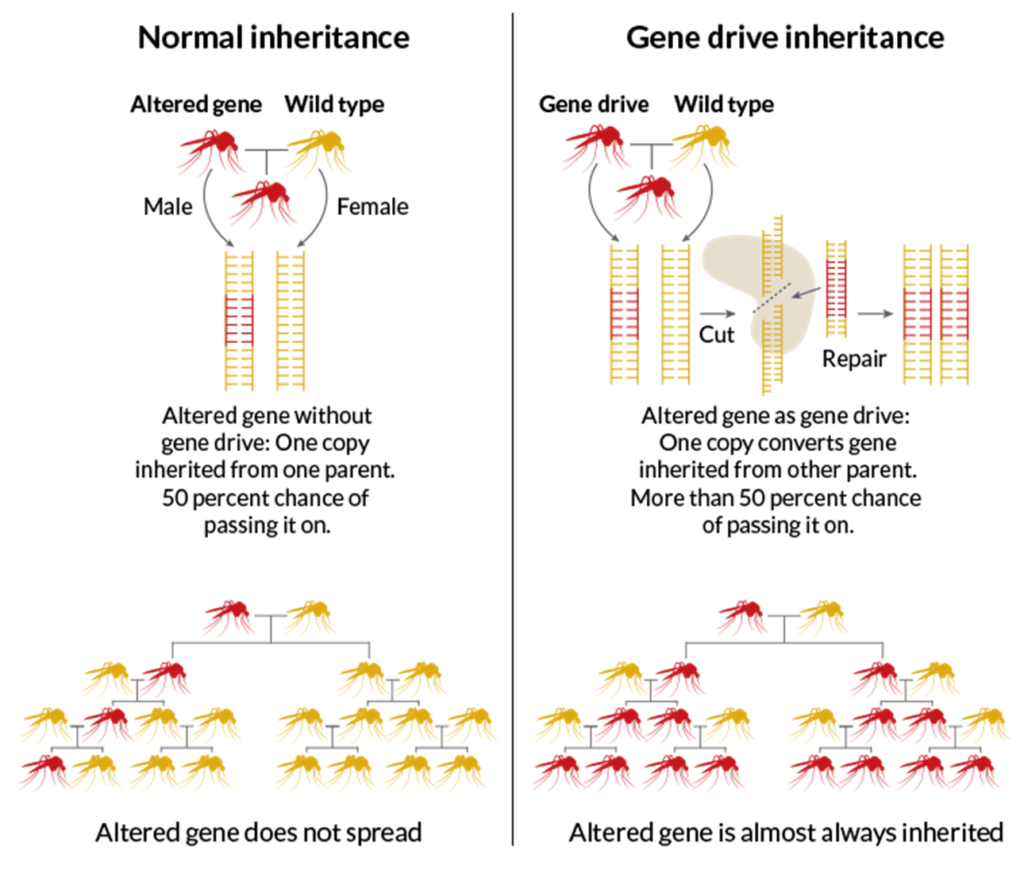
gene drive could cause evolutionary chaos
A future without mosquitoes is also a future without malaria, Zika virus, chikungunya virus, dengue fever — the list goes on and on. Without these insects to spread disease, lives would be saved around the world.
CRISPR could help us get there. But what seems like a good idea from the surface could have catastrophic consequences from an evolutionary standpoint.
In general, each gene has a 50% chance of being passed down. But gene drive offers a way to make a certain inheritance outcome more favorable.
If scientists alter gene drive with genetic-editing techniques, they could create a fast track for evolution.
Credit: E. OTWELL AND M. TELFER, ScienceNews.org
In December 2016, researchers at Imperial College London envisioned a way to eliminate the whole mosquito population that carries the malaria parasite. This would be achieved by editing genes using the CRISPR system and adding in a DNA sequence that would ensure biased inheritance in mosquito embryos. As these mosquitoes developed and matured into adults, they would mate with other wild mosquitoes in the population, and eventually stop carrying malaria.
But what seems like such a great idea in the short-term could disrupt the natural balance of wild ecosystems, altering the course of natural evolution. The domino effect of consequences through ecosystems is impossible to predict.
Genome editing as a Weapon of mass destruction?
In the February 2016 report of Worldwide Threat Assessment of the US Intelligence Community prepared by James Clapper, Director of National Intelligence, “Genome Editing” was listed as a potential natural security threat outlined under Weapons of Mass Destruction and Proliferation.
Genetic watchdog groups like EcoNexus and ETC Group caution that gene editing can be misused. CRISPR could give rise to a new generation of bioweapons through the engineering of new pathogens or serve as inspiration for bioterrorists. If an insect can carry and deliver toxins, this technology could be weaponized by rogue groups.
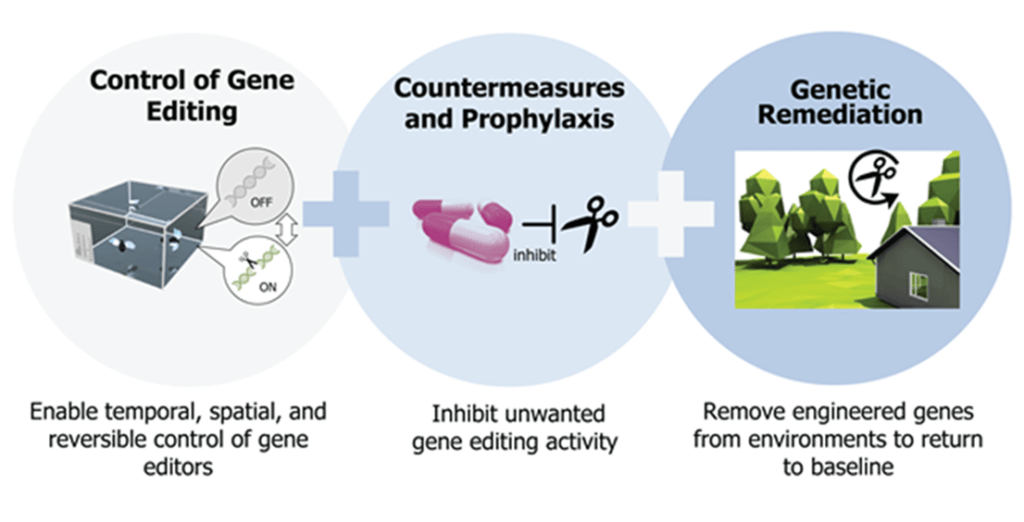 Image: Defense Advanced Research Projects Agency (DARPA)’s Safe Genes program. Credit: DARPA
Image: Defense Advanced Research Projects Agency (DARPA)’s Safe Genes program. Credit: DARPA
Defense Advanced Research Projects Agency (DARPA), an agency of the Department of Defense, is already preparing for such scenarios and developing antidotes as they work to find solutions that could reverse harmful gene drive effects.
Patent wars
Since initial papers on CRISPR and its applications were published in 2012, scientists Jennifer Doudna of UC Berkeley and Feng Zhang of Broad Institute have been racing to patent this technology.
The contentious fight for a key CRISPR-Cas9 patent was finally awarded to Zhang at Broad Institute by the US Patent and Trademark Office in February 2017. However, the European Patent Office (EPO) just revoked one of Broad’s patents this month.
Going forward, the status of key CRISPR-related patents and their impact on research in this field will be something to watch. With plenty of patent families up in the air, the future battles for CRISPR patents will heat up.
Closing remarks
The landscape of gene editing could look completely different in 100, 50, or even 10 years.
In the future, it might be standard to tweak or design genes in plants, animals, and even human beings, irrevocably affecting the gene pool and the course of evolution.
While some ideas presented above may seem far-fetched at this the moment, that could easily change. After all, CRISPR isn’t an expensive, inaccessible form of technology. It’s available and in use now. From farmers to researchers, CRISPR will make its impact in our society.